Therapeutic Alignment for Excluded and Refractory Patient Populations
Strategic Preview (Public Summary)
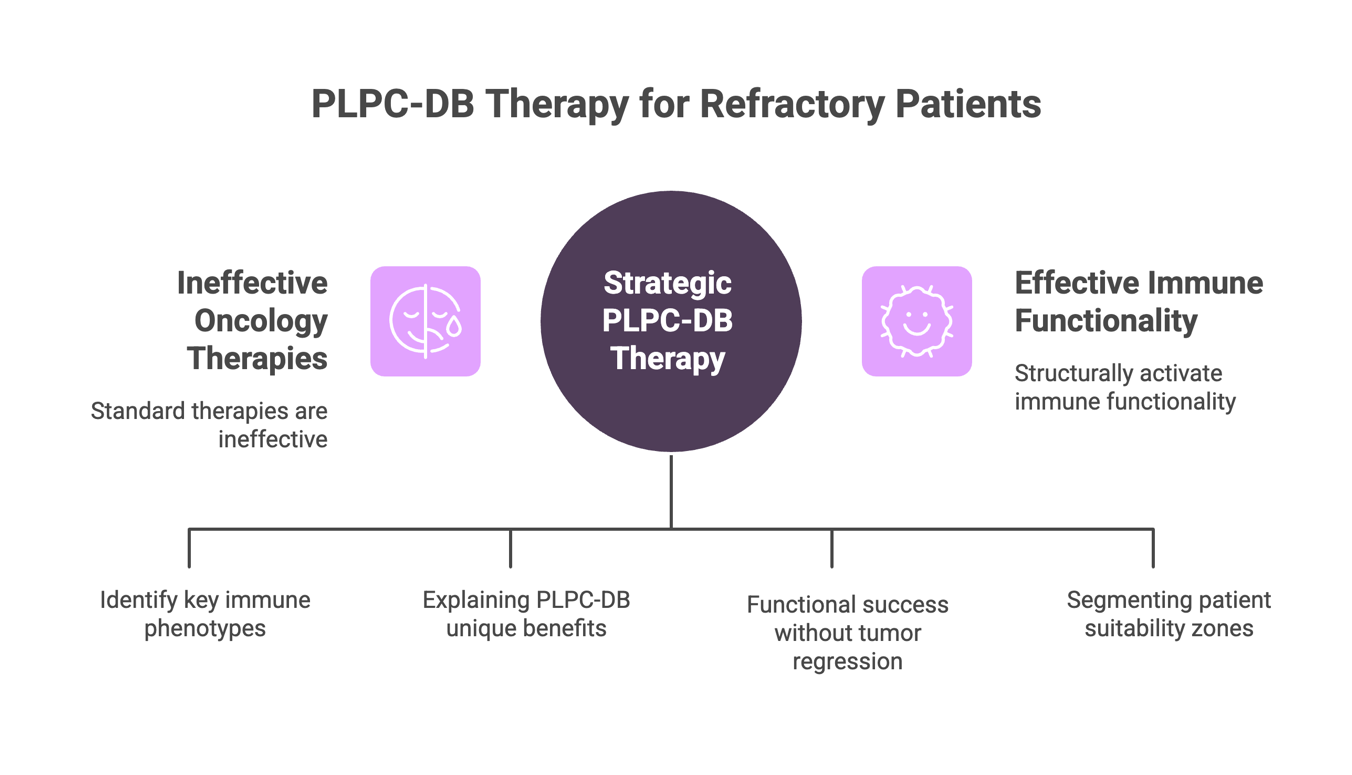
PLPC-DB is not designed to compete with standard oncology therapies—it is designed to intervene precisely where they no longer work.
From elderly patients with immunosenescence, to post-chemotherapy immune exhaustion, to tumors with checkpoint resistance or IL-10↑/HLA-DR↓/CD69⁻ profiles, PLPC-DB structurally activates immune functionality in contexts that conventional therapies are unable to reach.
This section outlines the patient archetypes who benefit most from PLPC-DB, based on biological feasibility, immunological markers, and operational limitations.
🔒 Why Is This Section Restricted?
The full content is restricted due to the high strategic value of the positioning data it contains. Specifically:
- It presents a logic of therapeutic substitution, positioning PLPC-DB as the sole viable immunological alternative in patient populations that have been systematically excluded from all current immunotherapies. Public access could be misinterpreted as overreach or used by competitors to reshape their own off-label arguments.
- It identifies immune phenotypes (e.g., IL-10↑, CD69⁻, HLA-DR↓) that justify PLPC-DB deployment without conventional indications. This logic is central to the platform’s non-receptor-based mechanism, and exposing it undermines proprietary differentiation.
- It includes a clinical value statement explaining why PLPC-DB can be used without systemic lymphocyte functionality—dismantling the core assumptions of current IO therapies (CAR-T, ICIs, TILs). This framework is highly sensitive from a regulatory and licensing standpoint.
- It supports
clinical–tumor dissociation models
documenting that therapeutic success can be functional and immunological even in the absence of tumor regression. Publicizing this alters standard oncology evaluation frameworks.
- It includes graphical stratification of patient populations, indicating suitability zones. This segmentation matrix is part of the licensing argumentation for regional application, and must remain controlled during negotiation phases.
In short, this page reveals not just who can benefit—but why no one else can serve them.
That is proprietary clinical territory.
Institutional Contact – Immediate Action
Request Access to CTD, STIP, or Due Diligence Files
To request confidential access to the full technical documentation—including regulatory modules, STIP records, SAP reports, and strategic materials—submit your request using the form below or via email.
WebDoc v2.1 – June 2025
